
J. Pablo Velazquez-Martin MD
A novel anti-VEGF antibody biopolymer conjugate currently undergoing clinical trials aims to significantly reduce the burden of treatment associated with intravitreal injections by delivering enhanced efficacy and ocular durability, according to J. Pablo Velazquez-Martin MD.
“This new antibody biopolymer conjugate (ABC) aims to improve real-world outcomes of anti-VEGF therapy, which are currently restricted by treatment burden and insufficient durability of existing anti-VEGF agents,” he told delegates attending the 9th EURETINA Winter Meeting in Prague.
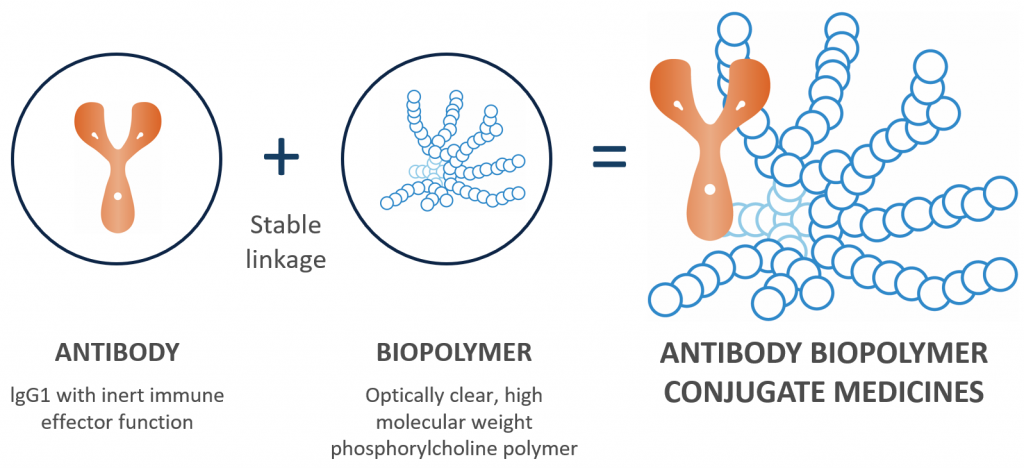
KSI-301 is an antibody stably linked to a biopolymer with a high molecular weight. The combination belongs to a new class of molecules: antibody biopolymer conjugate, or ABC, medicines. KSI-301 was designed to optimise both size and formulation strength to improve durability in the eye, said Dr Velazquez-Martin.
“This agent clinically targets VEGF using an intravitreal injection in an optically clear solution with no ocular residues. However, unlike current anti-VEGF agents, KSI-301 is designed for ocular durability, fast systemic clearance and improved bioavailability, biocompatibility, and stability,” he said.
In a recent phase I study, KSI-301 was tested for safety on previously-treated or naïve subjects with diabetic macular oedema (DME). The open-label, single ascending dose study at five different centres in the United States included nine study eyes, three per dosing cohort, who received a single dose of KSI-301 (1.25mg, 2.5mg or 5mg) via a standard intravitreal injection and were then followed for 12 weeks.
In terms of safety outcomes, no drug-related adverse events, intraocular inflammation or dose-limiting toxicities were observed.
“The absence of intraocular inflammation is very important for a new molecule. The study eyes all showed optically clear media after each injection. No anti-drug antibodies were detected in any patient and all dose levels were well tolerated,” he said.
Patients also showed improvements in vision and retinal thickness after single-dose administration of KSI-301 over the 12-week follow-up period.
“Rapid-onset, high-magnitude improvements in best-corrected visual acuity with corresponding reductions in macular thickness on OCT were observed at all dose levels and the median improvements were durable out to 12 weeks,” noted Dr Velazquez-Martin.
Further studies of the drug are planned in the near future, including an ongoing phase Ib trial evaluating multiple doses in treatment-naïve wet AMD, DME and retinal vein occlusion (RVO) and a phase II trial in treatment-naïve wet AMD with dosing as infrequently as every 20 weeks after the initial loading phase.
“The objective is ultimately to make this the ‘go-to drug’ for induction and maintenance therapy of retinal vascular diseases,” concluded Dr Velazquez-Martin.
J. Pablo Velazquez-Martin: pablov@kodiak.com


 J. Pablo Velazquez-Martin MD
J. Pablo Velazquez-Martin MD