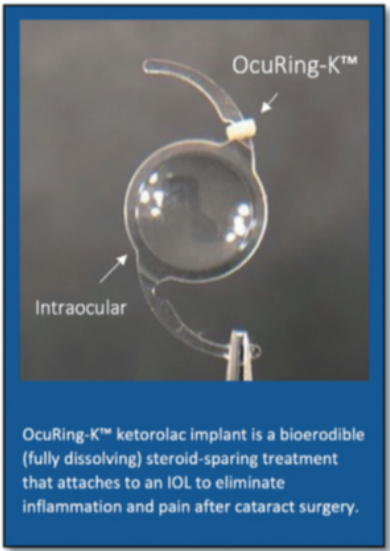
Intraocular delivery of ketorolac with the haptic-affixed OcuRing- KTM (Layerbio, Inc.) reduces inflammation and pain from cataract surgery without untoward effects and without modification of surgical technique, according to the results of a Phase 1I open-label trial presented by Kenneth J. Mandell MD, PhD at the ARVO 2021 Congress.
“These findings support the potential of the OcuRing-K implant as a safe and effective alternative to NSAID eye drops for use in cataract surgery,” said Dr Mandell, founder and CEO of Layerbio Inc., Lexington, Massachusetts, United States.
The study involved five patients who underwent cataract extraction and implantation of an intraocular lens (IOL) with the OcuRing-K. Dr Mandell and his associates evaluated the patients postoperatively on day one, day seven, and day 28. They assessed inflammation using the standardisation of uveitis nomenclature (SUN) scale anterior chamber cell (ACC) score and postoperative pain using a visual analogue scale.
He noted that the new bio-erodible ring is designed for attachment to an IOL’s haptic just prior to loading the lens into its injection cartridge. The ring’s elastic properties enable its use with any standard IOL. The implant releases the nonsteroidal anti-inflammatory drug (NSAID) ketorolac for 14 days.
Dr Mandell pointed out that ketorolac is the active ingredient of several ophthalmic medications currently FDA-approved for use after cataract surgery. Moreover, the OcuRing-K’s intraocular mode of drug delivery avoids the risk of corneal complications that may occur with the topical administration of NSAIDs in certain high-risk patients.
SAFE EFFECTIVE AND EASY TO USE
In their study, the surgeon successfully implanted the IOL with the OcuRing-K in five patients without complication. In addition, slit lamp examination showed that in all five patients the IOLs were centred on the visual axis without tilt. There was also no evidence of contact between the NSAID implant the iris, Dr Mandell said.
Furthermore, the mean postoperative ACC scores were 0.6 and 0.4 at days one and day seven, respectively, and no ACC was observed in any subjects by day 28. Moreover, all patients were pain-free at all follow-up assessments, and none of the patients required rescue therapy with topical anti-inflammatory medication. There were no treatment-related adverse events.
Among the surgeon’s observations were that the OcuRing-K was compatible with the standard IOLs and injector systems most commonly used in cataract surgery. In addition, the OcuRing-K’s presence on the IOL’s haptic did not interfere with the insertion or unfolding of the IOL within the capsular bag or with the rotation and centring of the IOL, he emphasised.
Kenneth J. Mandell MD, PhD: ken@layerbio.com