Corneal Therapeutics
Restoring Corneal Transparency
Developing novel cell-based therapies for limbal stem cell deficiency and stromal injury.

Cheryl Guttman Krader
Published: Friday, September 30, 2022
Extracellular vesicles (EVs) derived from human corneal stromal stem cells (hCSSCs) could form the basis for affordable therapies to prevent or treat stromal scarring in injured corneas, according to Sophie X Deng MD, PhD during a symposium on cell-based therapies for ophthalmic diseases. She described the work addressing limitations surrounding autologous LSC transplantation for LSCD.
Members of the Global Consensus on Management of LSCD Working Group concluded autologous LSC transplantation using the least amount of donor tissue is the preferred method for surgical treatment of unilateral or subtotal bilateral LSCD. They identified the use of ex vivocultivated autologous LSCs as one such approach. However, a drawback of existing expansion methods for obtaining the transplant material was the use of animal products.
To overcome this issue, researchers in Dr Deng’s laboratory, led by Sheyla Gonzalez PhD, developed a xenobiotic-free and feeder-free manufacturing process for LSC expansion in which the limbal biopsy is grown directly on denuded amniotic membrane.
Dr Deng and colleagues also addressed another limitation in LSCD management—the absence of a standardised objective system for diagnosing the disease and grading its severity.
Targeting this unmet need, Dr Deng and colleagues developed a quantitative diagnostic and staging system for LSCD that grades the severity of the condition with a numerical score. The system is a composite of a clinical score and findings from in vivo imaging. The clinical score considers the extent of limbal involvement, extent of corneal involvement, and presence of central axis involvement. The imaging parameters are central cornea basal epithelial cell density, corneal epithelial thickness, cell morphology, and corneal subbasal nerve fibre density.
PHASE I STUDY UNDERWAY
The safety, feasibility, and efficacy of treating LSCD using autologous LSCs cultivated with the xenobiotic-free manufacturing process are under investigation in an ongoing phase 1 clinical trial. As a second objective, the study aims to validate the LSCD diagnostic criteria and quantitative staging system.
The study has a randomised, controlled, open-label design and plans to enrol 20 subjects with severe to total LSCD in one eye. Fifteen patients will receive LSC treatment, and five assigned to a scleral lens control group.
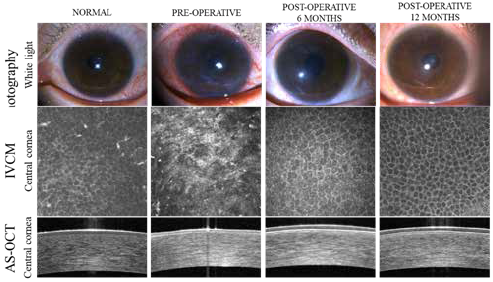
“Preliminary results show so far that six limbal stem cell grafts manufactured under xenobiotic-free and feeder-free conditions from six limbal biopsies have been successfully transplanted. In addition, there have been no serious adverse events associated with the transplanted cultivated LSCs, and most patients experienced dramatic improvements in subjective symptoms and vision too,” Dr Deng said. The corneal epithelial cell layer with normal cell morphology, basal cell density, and epithelial thickness could be restored after cLSC treatment (as shown in the figure). “This finding is very exciting because this is the first time biological function of transplanted LSCs has been demonstrated and quantified at the cellular level in patients using a set of standardised criteria,” she added. These criteria can be used in future clinical trials as standardised outcome measures that enable objective comparison of efficacy of different LSC therapies, which has not been possible.
“We are still actively recruiting participants for the study. Please refer patients with unilateral LSCD.”
STROMAL SCAR TREATMENT
The development of hCSSC-derived extracellular vesicle-based therapy for corneal stromal scarring holds interest for overcoming the severe shortage of corneal tissue for transplantation. Dr Deng credited James Funderburgh PhD from the University of Pittsburgh (Pennsylvania, US) for pioneering this field, as his group first demonstrated that CSSCs— which are mesenchymal stem cells within the limbus—have antifibrotic activity.
“In animal models with corneal injury induced by mechanical wounding, Dr Funderburgh and colleagues showed the application of CSSCs reduced scar formation. Phase 1 clinical trials using CSSCs are now being conducted in India,” Dr Deng said.
Dr Funderburgh’s subsequent work validated his hypothesis that EVs from the CSSCs, specifically microRNAs transported in the vesicles, were the key mediator of corneal regeneration. The collaborative work of Dr Funderburgh and Dr Deng further showed that a specific subset of microRNAs might be responsible for the anti-fibrotic function of EVs.
“The evidence indicates EVs from CSSCs are efficient in their ability to reduce corneal scarring and seem to be more stable, safer, and more accessible a treatment for corneal scarring than CSSCs because the EVs can be formulated as an off-the-shelf product,” said Dr Deng.
“However, the CSSCs have a limited replicative lifespan and cannot produce enough EVs for clinical use.”
To overcome this “bottleneck” that prevents the necessary scaling up of EV production, Dr Deng and colleagues set out to create an immortalised CSSC line that could provide an unlimited EV source. Their efforts were successful, as characterisations of the immortalised cells showed the cells maintained the morphology of primary CSSCs, expressed the same cell surface antigens, retained a similar molecular signature, and retained the multipotent differentiation ability into adipogenic, osteogenic, and chondrogenic lineages of the primary CSSCs.
Subsequent research performed in a mouse model of mechanical stromal wounding demonstrated EVs derived from the immortalised CSSCs reduced corneal scarring.
“This work is proof of concept that immortalised CSSCs can produce a large amount of functional EVs and for the feasibility of low-cost, EV-based therapy,” Dr Deng said.
Dr Deng presented this research at ARVO 2022 in Denver, Colorado, US.
Sophie Deng MD, PhD is the director of the Cornea Biology Laboratory, Joan and Jerome Snyder Chair in Cornea Diseases, and co-Chief, Cornea and Uveitis Division at the UCLA Stein Eye Institute, Los Angeles, California, US.
Latest Articles
Towards a Unified IOL Classification
The new IOL functional classification needs a strong and unified effort from surgeons, societies, and industry.
The 5 Ws of Post-Presbyopic IOL Enhancement
Fine-tuning refractive outcomes to meet patient expectations.
AI Shows Promise for Meibography Grading
Study demonstrates accuracy in detecting abnormalities and subtle changes in meibomian glands.
Are There Differences Between Male and Female Eyes?
TOGA Session panel underlined the need for more studies on gender differences.
Simulating Laser Vision Correction Outcomes
Individualised planning models could reduce ectasia risk and improve outcomes.
Need to Know: Aberrations, Aberrometry, and Aberropia
Understanding the nomenclature and techniques.
When Is It Time to Remove a Phakic IOL?
Close monitoring of endothelial cell loss in phakic IOL patients and timely explantation may avoid surgical complications.
Delivering Uncompromising Cataract Care
Expert panel considers tips and tricks for cataracts and compromised corneas.
Organising for Success
Professional and personal goals drive practice ownership and operational choices.