Slowing glaucoma progression
Blocking connexins in retinal astrocytes may help protect the optic nerve

Howard Larkin
Published: Tuesday, October 1, 2019
 A growing body of research suggests that connexin 43 (Cx43), a transmembrane cellular protein that facilitates chemical signalling between cells, may play an important role in glaucoma progression. Blocking its effects in injured and diseased retinal tissues may slow neurodegeneration, Helen Danesh-Meyer MBChB, MD, PhD, told the Glaucoma Subspecialty Day at the 2019 American Society of Cataract and Refractive Surgery Annual Meeting in San Diego, USA.
Recent insights into the role of astrocytes and Cx43 in creating an environment that perpetuates neurodegeneration could revolutionise the understanding of glaucoma, possibly answering questions including why it often progresses even in eyes with well-controlled intraocular pressure (IOP), said Dr Danesh-Meyer, who is Sir William and Lady Stevenson Professor of Ophthalmology at the New Zealand Eye Centre, Auckland, NZ.
“We’ve been focused on the retinal ganglion cell in our glaucoma research. … To take glaucoma treatment to the next step we need to consider the environment, the astrocytes that surround RGCs,” Dr Danesh-Meyer said.
CHANGING THE NEURONAL ENVIRONMENT
Astrocytes surround axons throughout the optic nerve head and make up much of the neuroglia, which is thought to persist as neurons die in glaucoma, possibly explaining the “floor effect” in nerve fibre layer thinning seen in advanced cases. Astrocytes support cell-cell communication, microenvironment control, “glio” transmitters, neuroplasticity, neuroinflammation and long-range information exchange.
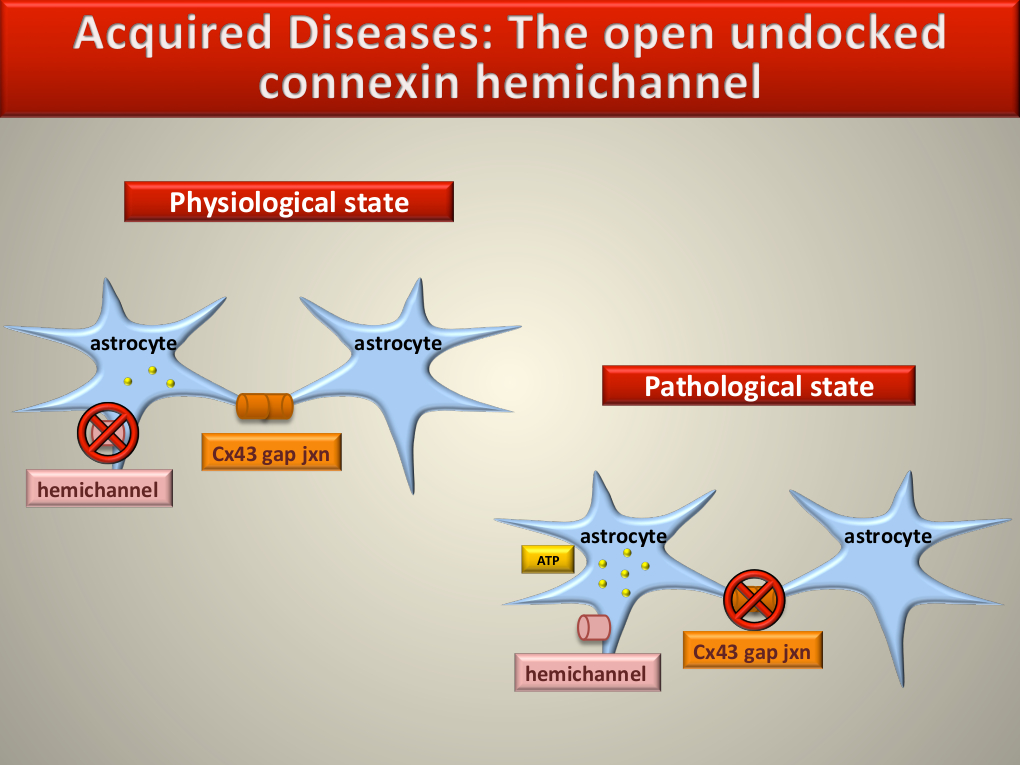
In their normal physiological state, astrocytes communicate in part by passing chemical and electrical signals across cell gap junctions bridged by bundles of six Cx43 molecules known as connexons. Connexons in adjoining cells dock with each other, forming channels that open to allow chemical signalling while undocked connexon hemichannels remain closed, Dr Danesh-Meyer explained.
In a pathological state the situation is reversed, Dr Danesh-Meyer noted. In addition to stressing neurons and RGCs, trauma, ischaemia and mechanical stress, including high IOP, activate astrocytes. This causes their gap junction channels to disassociate and close, and opens undocked hemichannels, releasing signalling compounds, notably adenosine triphosphate (ATP), into the extracellular space. Extracellular ATP further stresses neurons and RGCs, and activates astrocytes resulting in astrocytosis in a self-reinforcing cycle.
Dr Danesh-Meyer and colleagues demonstrated the relationship between optic nerve injury and astrocytosis in a rat model of acute injury. Elevating IOP to 120mmHg for 60 minutes followed by reperfusion of the retina increased Cx43 protein associated with astrocytosis (Danesh-Meyer et al. Brain 2012; 135:506-520).
The evidence is strengthened by post-mortem studies of glaucomatous human eyes, in which increased astrocyte activation and upregulation of Cx43 were found in the laminar cribrosa and retina compared with controls (Ker NM et al. J Clin Neuroscience. 2011; 18:102-108).
“Stress on the retina, whether it is an optic nerve crush or ischaemia or glaucoma or diabetic retinopathy, produces astrocytic activation that seems to occur following injury,” Dr Danesh-Meyer said.
“Leaky” Cx43 channels are also associated with non-ocular neuropathies, including Alzheimer’s (Frontiers in Cellular Neuroscience. 27 July 2015), as well as stroke, trauma, infection and chronic conditions such as Huntington’s disease, Parkinson’s, MS, epilepsy and migraine, she noted.
HELPFUL OR HARMFUL?
So the question becomes: Is Cx43 and astrocyte activation neuroprotective or harmful? Dr Danesh-Meyer’s research suggests the latter.
Following up her study of retinal damage to rats, administering a Cx43 hemichannel blocker reduced vessel leakage and significantly reduced RGC loss seven and 21 days post-ischaemia (p<0.05) (Danesh-Meyer et al. Brain 2016).
“This can lead to neurorescue, which suggests Cx43 and astrocyte activation is a negative process that leads to perpetuation of damage.”
A study using a Cx43 antisense compound in an animal spinal crush study reinforces the evidence, showing less scarring, inflammation and astrocytic activation, leading to improved motion in treated subjects (Mol Cell Neurosci 2008; 39:152-160).
Future research should focus on the role of connexins in chronic versus acute glaucoma, and in humans rather than animals. Whether treatment is effective when the disease process is well advanced or mild, and whether down regulation of hemichannel opening can be combined with other neuroprotective strategies are key unanswered questions, Dr Danesh-Meyer said.
“We’ve focused on the neuron. Perhaps we’ve neglected the environment in which that neuron lives.”
A growing body of research suggests that connexin 43 (Cx43), a transmembrane cellular protein that facilitates chemical signalling between cells, may play an important role in glaucoma progression. Blocking its effects in injured and diseased retinal tissues may slow neurodegeneration, Helen Danesh-Meyer MBChB, MD, PhD, told the Glaucoma Subspecialty Day at the 2019 American Society of Cataract and Refractive Surgery Annual Meeting in San Diego, USA.
Recent insights into the role of astrocytes and Cx43 in creating an environment that perpetuates neurodegeneration could revolutionise the understanding of glaucoma, possibly answering questions including why it often progresses even in eyes with well-controlled intraocular pressure (IOP), said Dr Danesh-Meyer, who is Sir William and Lady Stevenson Professor of Ophthalmology at the New Zealand Eye Centre, Auckland, NZ.
“We’ve been focused on the retinal ganglion cell in our glaucoma research. … To take glaucoma treatment to the next step we need to consider the environment, the astrocytes that surround RGCs,” Dr Danesh-Meyer said.
CHANGING THE NEURONAL ENVIRONMENT
Astrocytes surround axons throughout the optic nerve head and make up much of the neuroglia, which is thought to persist as neurons die in glaucoma, possibly explaining the “floor effect” in nerve fibre layer thinning seen in advanced cases. Astrocytes support cell-cell communication, microenvironment control, “glio” transmitters, neuroplasticity, neuroinflammation and long-range information exchange.
In their normal physiological state, astrocytes communicate in part by passing chemical and electrical signals across cell gap junctions bridged by bundles of six Cx43 molecules known as connexons. Connexons in adjoining cells dock with each other, forming channels that open to allow chemical signalling while undocked connexon hemichannels remain closed, Dr Danesh-Meyer explained.
In a pathological state the situation is reversed, Dr Danesh-Meyer noted. In addition to stressing neurons and RGCs, trauma, ischaemia and mechanical stress, including high IOP, activate astrocytes. This causes their gap junction channels to disassociate and close, and opens undocked hemichannels, releasing signalling compounds, notably adenosine triphosphate (ATP), into the extracellular space. Extracellular ATP further stresses neurons and RGCs, and activates astrocytes resulting in astrocytosis in a self-reinforcing cycle.
Dr Danesh-Meyer and colleagues demonstrated the relationship between optic nerve injury and astrocytosis in a rat model of acute injury. Elevating IOP to 120mmHg for 60 minutes followed by reperfusion of the retina increased Cx43 protein associated with astrocytosis (Danesh-Meyer et al. Brain 2012; 135:506-520).
The evidence is strengthened by post-mortem studies of glaucomatous human eyes, in which increased astrocyte activation and upregulation of Cx43 were found in the laminar cribrosa and retina compared with controls (Ker NM et al. J Clin Neuroscience. 2011; 18:102-108).
“Stress on the retina, whether it is an optic nerve crush or ischaemia or glaucoma or diabetic retinopathy, produces astrocytic activation that seems to occur following injury,” Dr Danesh-Meyer said.
“Leaky” Cx43 channels are also associated with non-ocular neuropathies, including Alzheimer’s (Frontiers in Cellular Neuroscience. 27 July 2015), as well as stroke, trauma, infection and chronic conditions such as Huntington’s disease, Parkinson’s, MS, epilepsy and migraine, she noted.
HELPFUL OR HARMFUL?
So the question becomes: Is Cx43 and astrocyte activation neuroprotective or harmful? Dr Danesh-Meyer’s research suggests the latter.
Following up her study of retinal damage to rats, administering a Cx43 hemichannel blocker reduced vessel leakage and significantly reduced RGC loss seven and 21 days post-ischaemia (p<0.05) (Danesh-Meyer et al. Brain 2016).
“This can lead to neurorescue, which suggests Cx43 and astrocyte activation is a negative process that leads to perpetuation of damage.”
A study using a Cx43 antisense compound in an animal spinal crush study reinforces the evidence, showing less scarring, inflammation and astrocytic activation, leading to improved motion in treated subjects (Mol Cell Neurosci 2008; 39:152-160).
Future research should focus on the role of connexins in chronic versus acute glaucoma, and in humans rather than animals. Whether treatment is effective when the disease process is well advanced or mild, and whether down regulation of hemichannel opening can be combined with other neuroprotective strategies are key unanswered questions, Dr Danesh-Meyer said.
“We’ve focused on the neuron. Perhaps we’ve neglected the environment in which that neuron lives.”
Latest Articles
Nutrition and the Eye: A Recipe for Success
A look at the evidence for tasty ways of lowering risks and improving ocular health.
New Award to Encourage Research into Sustainable Practices
Sharing a Vision for the Future
ESCRS leaders update Trieste conference on ESCRS initiatives.
Extending Depth of Satisfaction
The ESCRS Eye Journal Club discuss a new study reviewing the causes and management of dissatisfaction after implantation of an EDOF IOL.
Conventional Versus Laser-Assisted Cataract Surgery
Evidence favours conventional technique in most cases.
AI Scribing and Telephone Management
Automating note-taking and call centres could boost practice efficiency.
AI Analysis and the Cornea
A combination of better imaging and AI deep learning could significantly improve corneal imaging and diagnosis.
Cooking a Feast for the Eyes
A cookbook to promote ocular health through thoughtful and traditional cuisine.
Need to Know: Spherical Aberration
Part three of this series examines spherical aberration and its influence on higher-order aberrations.
Generating AI’s Potential
How generative AI impacts medicine, society, and the environment.