Colour blindness
Research supports feasibility of gene therapies for severely disabling colour blindness

Roibeard O’hEineachain
Published: Monday, April 1, 2019
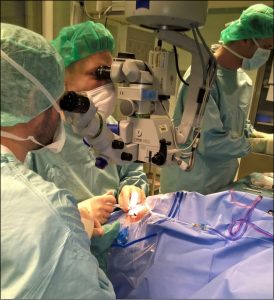 The world’s first gene therapy for achromatopsia:
Tübingen on 11.11.2015 by (left to right)
Prof Fischer, Prof Karl Ulrich Bartz-Schmidt
and Mr Apostolos Bezirgiannidis
The world’s first gene therapy for achromatopsia:
Tübingen on 11.11.2015 by (left to right)
Prof Fischer, Prof Karl Ulrich Bartz-Schmidt
and Mr Apostolos Bezirgiannidis